

In an isoelectronic series, ions have the same number of electrons.Ions increase in size as you go down a column.Electron-electron repulsion causes the electrons to spread out more in space.Anions (-) are larger than their parent atoms.Electrons are removed from the outer shell.Cations (+) are smaller than their parent atoms.Example: Which element would have the larger atomic radius, Ar or Br?.
Sizes of Atoms Bonding atomic radius tends to… …decrease from left to right across a row …increase from top to bottom of a column Within a period (row), the atomic radius tends to decrease as we move from left to right.Within a group (column), the atomic radius tends to increase from top to bottom.The relative size (radius) of an atom of an element can be predicted by its position in the periodic table.What Is the Size of an Atom? The bonding atomic radius is defined as one-half of the distance between covalently bonded nuclei. Chemical and physical properties of the elements vary with their position in the periodic table.A group of atoms or ions that all contain the same number of electrons.N3-, O2-, F-, Ne, Na+, Mg2+, and Al3+ form an isoelectronic series.Isoelectronic:having the same number of electrons.8A H 2A He 3A 4A 5A 6A 7A Li Be B C N O F Ne Na Mg Al Si P S Cl Ar 7B 8B 8B 8B 1B 2B 3B 4B 5B 6B K Ca Sc Ti V Cr Fe Co Ni Cu Zn Ga Ge As Se Br Kr Mn Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Md No Lr Fm When atoms ionize, they form ions with the same number of electrons as the nearest (in atomic number) noble gas.
Krypton orbital diagram plus#
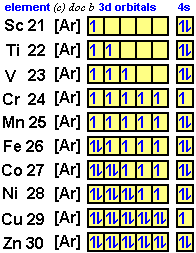
Previous noble gas: Ar (Z = 18) Extra electrons: 35 - 18 = 17 Period number: 4 So: Ar core plus 17 extra e- starting with 4s 4s23d104p5 Previous noble gas: Kr (Z = 36) Extra electrons: 38 (e of Sr) - 36 = 2 Period number of Sr: 5 So: Kr core plus 2 extra e- starting in 5s 5s2Įlectron Configuration Write the core electron configuration of Br (Z = 35). Add electrons starting in that “n” subshellĮlectron Configuration Write the core electron configuration of Sr (Z = 38).(This determines the value of n of the s subshell to start with when adding extra electrons) Determine the period that element is in.(= Atomic number of atom minus atomic number of noble gas) Determine how many additional electrons must be added beyond what that noble gas has.Find the Noble Gas that comes before the atom.The noble gas “core” can be used to write the electron configuration of an elementusing core notation: noble gas “core” + valence electronsĬore notation To write the electron configuration using the core notation:.An O2- ion has 8 protons and 10 electrons 1s22s22p6Įlectron Configuration Write the electron configuration of a krypton atom (Z = 36). 1s2 2s22p6 3s23p6 4s2 3d5Įlectron Configuration Example: Write the electron configuration of an O2- ion (Z = 8). use the “diagonal” diagram to help determine the filling orderĮlectron Configuration Example: Write the electron configuration of a Mn atom (Z = 25).add electrons to each subshell in the correct filling order until all electrons have been added.determine the number of electrons present.To determine the electron configuration of an atom (or ion) without first writing the orbital diagram:.The orbital diagram for an O atom: The electron configuration for an O atom: 1s22s22p4.If there are three electrons in a 2p subshell, we would write: 2p3 where the superscript (3) indicates the number of electrons in that subshell.The electron configuration tells the number of electrons found in each subshell.a particular arrangement of electrons in the orbitals of an atom.A short-hand notation is commonly used in place of orbital diagrams to describe the electron configuration of an atom.Drawing orbital diagrams gives information not only about the orbitals that are/have been filled but also about the number of unpaired electrons.Orbital Diagrams and Electron Configuration


 0 kommentar(er)
0 kommentar(er)
